
Depression
Latest News

Latest Videos
Shorts

CME Content
More News

Gus Alva, MD, reviews key 2025 psychiatry advances, including non-monoaminergic approaches to MDD and newer targets in schizophrenia care.

Flow Therapeutics announced approval of its Flow-100 tDCS therapy for depression, which could expand access to non-pharmacologic care and will be available in the US in H2 2026.

Adolescents acquiring smartphones at 12 showed increased risk for depression, obesity and sleep disturbances, according to a study of more than 10,000 youths.

Psychiatric comorbidities affect 25% of AD patients. Here, a check list on when to screen, when to refer, and how to prevent accumulated psychological burden in primary care.

A regular focus on mental heatlh during follow-ups with patients who have atopic dermatitis can be integrated simply with a few thoughtful questions.

Mental health apps show high initial uptake but moderate adherence, revealing key factors for improving user retention in clinical trials.
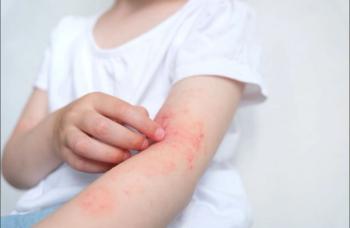
Controlling atopic dermatitis signs and symptoms early can alter social, emotional, and functional outcomes, sometimes dramatically.

Emotional red flags in AD, eg, sleep loss, isolation, hopelessness, often go unseen. Quick screening questions can surface the real burden individuals carry.

Growing up with AD carries lifelong emotional consequences. Clinicians who recognize these developmental risks can better support patients at every stage.

Up to 1 in 4 patients with atopic dermatitis face anxiety or depression. Primary care clinicians are key to identifying risk and supporting mental well-being.

Deep TMS becomes first FDA-cleared brain stimulation therapy for adolescents with treatment-resistant depression and is now available for ages 15-86 for MDD treatment.

As adjunctive treatment, lumateperone is associated with early symptom improvement, good tolerability, and provides a practical no-titration option for adults with persistent MDD.

Clinical and real-world data are on deck for lumateperone, seltorexant, and esketamine to treat major depressive disorder and treatment-resistant depression.
The ReCODE program targets metabolic, infectious, immune, vascular, and toxic exposures, elements that underlie both mood and cognition, study authors said.

Lipocene expects to announce interim safety data from the study this quarter and said the phase 3 program results will support the company's NDA in 2026.

Today's episode covers depression risk after chronic illness, vaginal estradiol safety, GLP-1 updates, cancer risk, and cardiovascular outcomes.

Teresa Lovins, MD, talked with Patient Care about suicidal ideation among patients and then focused on asking her colleagues to make their own mental health a priority.

The phone call can be short, but the outreach and contact are key after a patient with suicidal ideation is referred for emergency or inpatient support, Lovins says.

Family physician Teresa Lovins, MD, explains how primary care clinicians can determine when to manage depression and when urgent referral is needed.

Teresa Lovins, MD, describes the PHQ-9, GAD-7, and other brief screeners that help family physicians identify depression, anxiety, ADHD, and bipolar disorder in everyday practice.

Primary care clinicians see a higher share of visits for depression than mental health professionals largely because they know their patients so well, Lovins said.

Teresa Lovins, MD, highlights a study that showed a 14% increase in rates of safety planning within 2 weeks of a negative screening led to a 25% decrease in attempted suicides.

Teresa Lovins, MD, sees at least 1 or 2 patients a week who have suicidal ideation. Too many of them are at risk for an attempt within a month of those visits.

Mental health issues affect half the population at some point during a lifetime. Teresa Lovins, MD, says FPs are often the first to learn that "something is going on."
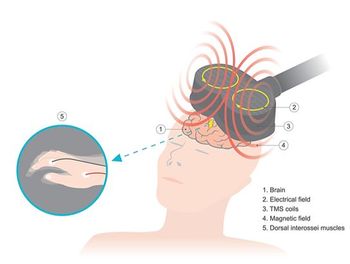
One-Day TMS condenses transcranial magnetic stimulation therapy treatment from a daily month-long process to 1 day, with rapid and durable results, NeuroStim says.

































































































































