Intralymphatic Immunotherapy Safely Reduces Allergy Symptoms
The emerging therapy may help overcome drawbacks of existing immunotherapies, such as treatment duration and poor adherence.
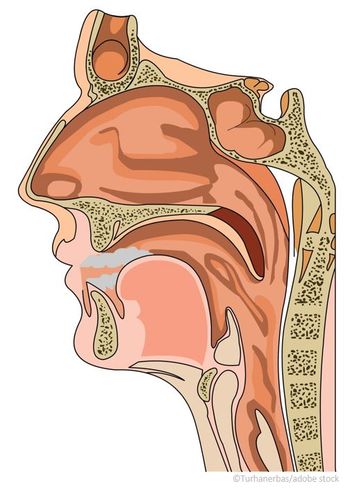
[[{"type":"media","view_mode":"media_crop","fid":"46815","attributes":{"alt":"","class":"media-image media-image-right","height":"319","id":"media_crop_1780714086997","media_crop_h":"0","media_crop_image_style":"-1","media_crop_instance":"5459","media_crop_rotate":"0","media_crop_scale_h":"0","media_crop_scale_w":"0","media_crop_w":"0","media_crop_x":"0","media_crop_y":"0","style":"float: right;","title":"©xrender/Shutterstock.com ","typeof":"foaf:Image","width":"336"}}]]Intralymphatic immunotherapy (ILIT) is a safe, effective treatment for pollen-induced rhinoconjunctivitis, markedly reducing the symptoms of seasonal allergy, according to a new study.
Allergen-specific immunotherapy (AIT) represents the only disease-modifying treatment for allergic diseases; it diminishes symptoms, improves quality of life, prevents new sensitizations, and reduces the risk of asthma development. The gold standard is subcutaneous immunotherapy (SCIT), but only 5% of patients undergo this therapy because of frequent injections, risk of adverse effects, and the long duration of treatment, according to the study authors, led by Terese Hylander of the Division of ENT Diseases at the Karolinksa Institutet in Stockholm, Sweden.
Sublingual immunotherapy (SLIT) is a more recent, non-invasive route of administration, but it is associated with reduced compliance as a result of the long period of self-medication required, they noted.
ILIT is an emerging form of immunotherapy that involves three injections of allergen over a period of 12 weeks. It “directs lower doses of allergen to the highly immunocompetent lymph node, in an effort to maximize chances for tolerance induction, while minimizing the risk for adverse effects,” the researchers stated.
The Karolinksa Institutet researchers have previously demonstrated that ILIT is a less time-consuming alternative to conventional SCIT. They recently expanded their research in a double-blind, placebo-controlled trial. The researchers randomized 36 patients (aged 18 to 65 years) with pollen-induced rhinoconjunctivitis to receive three intralymphatic inguinal injections of active allergen or placebo. They assessed clinical effects, safety, and circulating immunologic markers before, four weeks after treatment, and at the end of the consecutive pollen season.
They published their
The results show that patients receiving active ILIT experienced a significant improvement in self-recorded seasonal allergic symptoms, as compared to placebo. No moderate or severe reactions were recorded following ILIT.
A subgroup of patients who improved also demonstrated a reduction in nasal symptoms following nasal allergen provocation.
No changes in total IgE or IgG4 were found. However, the affinity of allergen specific IgG4 following active treatment was significantly increased, as compared to non-improved patients.
“In the present study, we have added a new cohort of study subjects and together they reinforce the hypothesis that ILIT is effective in reducing allergic symptoms and that it is not associated with severe adverse events. In addition, we highlight a role for increased allergen-specific IgG4 affinity in successful ILIT,” the researchers stated.
They noted that the 60% success rate of ILIT is comparable to the approximate 70% success rate for SCIT.
ILIT uses complex medical equipment and involves higher technical precision, as targeted nodes are relatively small (0.5–1.5 cm). Therefore, it can be difficult to penetrate the capsule surrounding the lymph node, which may lead to inadequate administration of allergen.
Another previous study found no clinical improvement using ILIT, but the Swedish researchers suggest that the Danish researchers had injected allergen every 2 weeks, which does not allow sufficient time for the development of antigen-specific immune responses.
“ILIT circumvents the drawbacks typically associated with SCIT, namely the frequent injections, high risk for adverse events, and long duration of treatment, and may therefore represent a novel lower-cost form of AIT,” they concluded
Source:
Hylander T, Larsson O, Petersson-Westin U, et al. I
Newsletter
Enhance your clinical practice with the Patient Care newsletter, offering the latest evidence-based guidelines, diagnostic insights, and treatment strategies for primary care physicians.