
Explore key clinical takeaways from FMX 2025, including updates on CGM, chronic cough, AAA screening, immunization, and more.

Explore key clinical takeaways from FMX 2025, including updates on CGM, chronic cough, AAA screening, immunization, and more.

This new episode covers global diabetes gaps, salt substitute use, influenza vaccine safety, vaginal estradiol, and preconception CT risks.
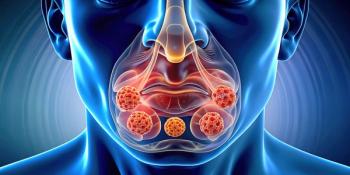
ACAAI 2025: Phase 3 results show dupilumab significantly reduced sinus opacification, nasal congestion, and polyp size in patients with AFRS.

Your daily dose of the clinical news you may have missed.

Nearly 40% of REDEFINE 1 participants taking antihypertensive medications reduced or discontinued their blood pressure drugs during the trial, Novo Nordisk reported.

Adults with diabetes often face obstacles to optimal disease management in the workplace. Use this checklist in the clinic to help them navigate the barriers.

New research suggests hormone therapy may increase autoimmune disease risk in postmenopausal women. Dr Jiang discusses early findings and clinical implications.

TMS 2025: Dr Marla Shapiro doesn't start perimenopausal therapy without an answer to the most important question: "What is your most bothersome symptom?"

Trump announces deals with Eli Lilly and Novo Nordisk slashing GLP-1 prices to as low as $150/month, expanding Medicare coverage starting mid-2026.

Oral semaglutide 25 mg improved glucose control and CV risk factors across weight loss categories in OASIS 4 post hoc analysis presented at ObesityWeek 2025.

As adjunctive treatment, lumateperone is associated with early symptom improvement, good tolerability, and provides a practical no-titration option for adults with persistent MDD.
Novo Nordisk’s STEP UP trial shows semaglutide delivers 21% weight loss and reduces key cardiovascular and metabolic risk factors in obesity management.

New longitudinal data from a Korean cohort suggest OSA may be an independent risk factor for cerebral small vessel disease.
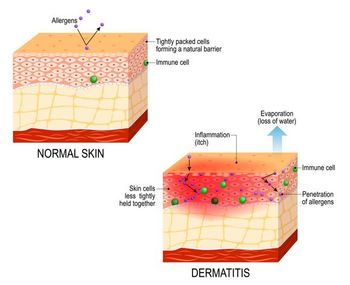
Novel AD therapies targeting OX40, IL-13, IL-36, IL-22, and IL-2 pathways show promise for disease modification and extended dosing intervals in phase 2-3 trials.

Global survey data highlight persistent stigma and self-management barriers for adults with diabetes in the workplace, with implications for clinical care.

The FDA approves a single monthly injection of mirikizumab-mrkz for ulcerative colitis, enhancing patient convenience and addressing bowel urgency effectively.

Patient preferences favor home-based tests but MDs do not, while cost barriers significantly impact follow-up completion, according to the 2 new analyses.

These 42 factors that affect blood glucose are in categories including food, activity, and biological influences. Perfect information to share with your patients with T2D.

Your daily dose of the clinical news you may have missed.