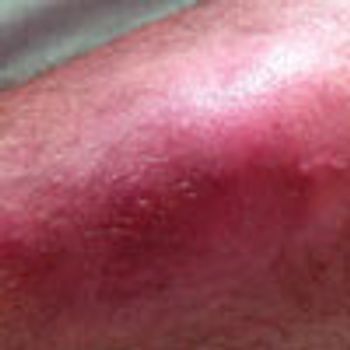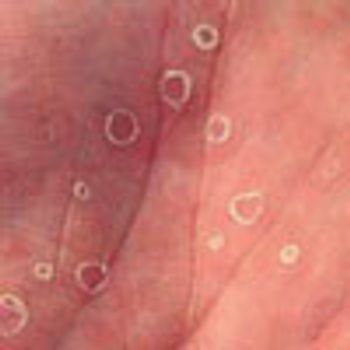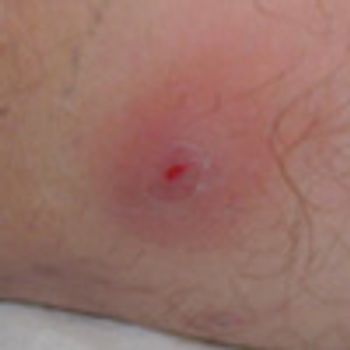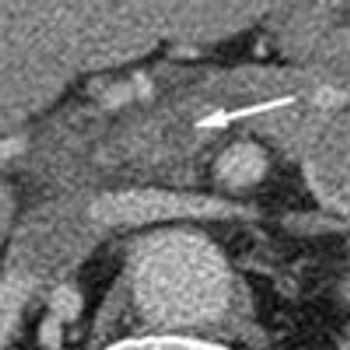
Acute abdominal pain, fever, and chills prompted a 51-year-old man to visit his local hospital twice in one week. On both visits, a clinical and laboratory workup was negative. He then presented to a tertiary care center with worsening symptoms. His history included hypertension and tobacco and alcohol use.