
A statin tops the list of most commonly prescribed Medicare drugs in most states. But in Alabama, it's Vicoodin, and the number of prescriptions written there exceeds the other 49 states.

A statin tops the list of most commonly prescribed Medicare drugs in most states. But in Alabama, it's Vicoodin, and the number of prescriptions written there exceeds the other 49 states.

Worldwide the report finds that hypertension, hypercholesterolemia, dietary risks, and air pollution are the leading causes of CVD which remains the leading cause of death globally.
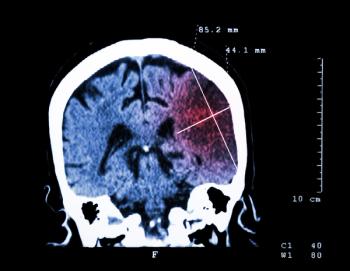
Similar reduction in risk was reported for both lobar and nonlobar intracranial hemorrhage with longer statin treatment associated with greater protection, according to Danish investigators.
Approved in 2020 as Nexletol, bempedoic acid as the first ATP citrate lyase inhibitor and oral non-statin therapy to meet the MACE-4 primary endpoint.

An EHR prompt to a physician alone or in combination with a previsit patient prompt both increased statin prescribing in primary care but a patient prompt alone did not.

Your daily dose of clinical news you may have missed.
The therapeutic impact of the dietary lifestyle intervention and the differential effects could have major implications for clinical care and policy, say study authors.

Your daily dose of clinical news you may have missed.
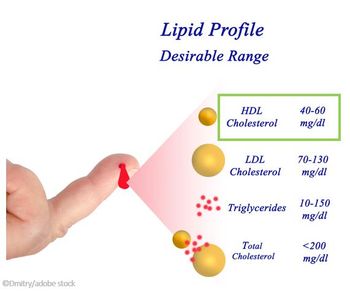
The analysis of a large biracial cohort found low levels of HDL-C linked to increased CHD risk in White but not Black adults and high HDL-C was not protective in either group.

Your daily dose of clinical news you may have missed.

The Mass General Brigham clinical intervention and study included 10 000 participants and resulted in significant reductions in SBP/DBP and LDL-C.

A 5-fold greater risk for all-cause or CVD-related death in persons diagnosed with T2D before age 40 years should be a call to action for enhanced screening, study authors state.

Physical performance scores used as a proxy for function in adults aged >65 years improved CVD risk prediction beyond traditional risk factors, study authors report.

In patients with hypertension, HDL-C >80 mg/dL was linked to a greater risk for CV events among men but not among women. Protective?

Social determinants of health, including education and income level and race/ethnicity, are drivers of declining cardiometabolic health in the US, says investigator Meghan O'Hearn.

Americans sitting on the cusp of cardiometabolic disease, ie, with prediabetes, prehypertension, overweight but not obesity, need intervention, now.

US prevalence of optimal cardiometabolic health has declined as poor levels have risen significantly over 2 decades. Investigator Meghan O'Hearn, MS, details the research.

Less than 7% of the US population has "optimal" cardiometabolic health, with declines over 20 years significantly affecting racial/ethnic populations.
Three-quarters of stroke patients in a Swiss registry had at least 1 undiagnosed risk factor at the time of the event, most commonly hyperlipidemia or hypertension.
US CV health is well below ideal, with 80% of adults scoring as only low or moderate in the first published study using AHA's new Life's Essential 8 algorithm.

Slide Show: The AHA added sleep duration to its Life's Simple 7 cardiovascular health construct and updated sections on diet, lipids, blood glucose and smoking.

A new meta-analysis questions the science demonstrating the link between statin-induced LDL-C lowering and improved CV, all-cause outcomes.
AHA 2021: New research showed MK-0616, an investigative oral PCSK9 inhibitor, was associated with a near 65% decrease in LDL-C.

AHA 2021: Study suggests remote hypertension and hyperlipidemia management programs may expand telehealth delivery, increase access to care, and reduce health inequities.

EASD 2021: For persons who have had obesity, returning to a healthy weight may reduce risk for hypertension and dyslipidemia, and modestly for diabetes.