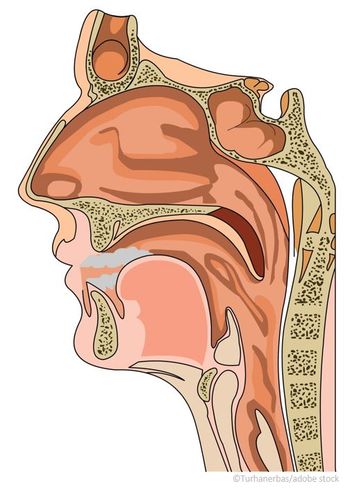
For patients with chronic sinusitis, disease exacerbations often require use of antibiotics and steroids, in addition to medical office visits, all of which impact quality of life.

Omalizumab Granted First FDA Indication for Children and Adults with Multiple Food Allergies

Takeda Announces Approval of First Oral Therapy for Eosinophilic Esophagitis

For patients with chronic sinusitis, disease exacerbations often require use of antibiotics and steroids, in addition to medical office visits, all of which impact quality of life.

Dupilumab is approved for the treatment of eosinophilic esophagitis in patients aged ≥12 years weighing at least 40 kgs.

Another new COVID-19 vaccine, the first and only once-daily amphetamine transdermal patch approved for the treatment of ADHD, and 5 more.

Tara Carr, MD, led a post hoc analysis of the NAVIGATOR phase 3 trial and presented data on tezepelumab efficacy in the vulnerable subgroup at the AAAAI 2022 meeting.
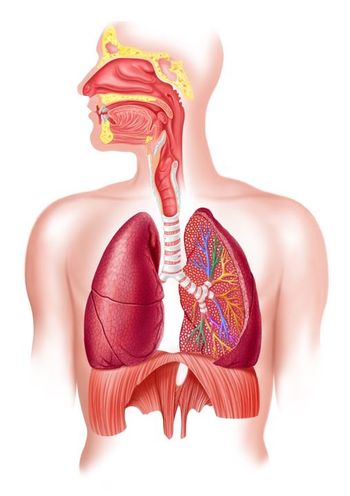
Tezepelumab, a TSLP inhibitor, reduced asthma exacerbation rates in patients with severe uncontrolled disease across a range of respiratory comorbidities.
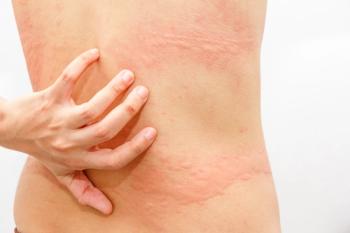
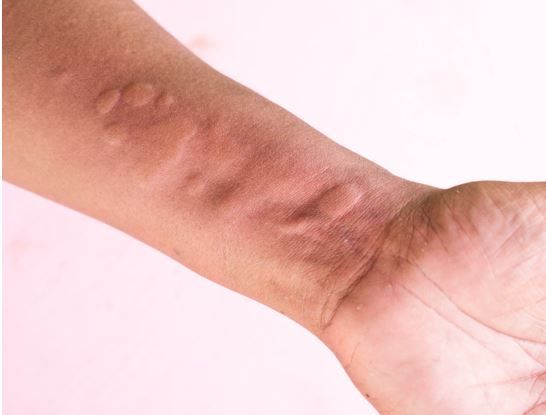
Dupilumab significantly reduced itch and hives in patients with H1 antihistamine-resistant chronic spontaneous urticaria, according to new research.
Research summaries on GINA revisions, a steroid-reduction algorithm, factors that predict asthma exacerbation risk, and more.

Optimal use of biologic therapy for severe uncontrolled asthma rests on understanding of the disease itself. Try this quick quiz to test your knowledge.
Adults with atopic disease and asthma had a 38% decreased risk of COVID-19 infection, according to a new UK study.

From obesity to pneumococcal disease, we have picked the top 6 FDA-approved drugs every primary care physician should know about from the second quarter.

The Asthma and Allergy Foundation of America recently released its annual Allergy Capitals™ report. Which cities are most challenging for patients with seasonal allergies?

Treatment for severe asthma may be enhanced using an online decision support tool that draws on expert treatment decisions for a wide variety of potential patient scenarios.

Dupilumab therapy for 4 weeks in adults with severe atopic dermatitis resulted in significant improvements in clinical outcomes with no adverse events reported.

The first and only drug to treat peanut allergy recently received approval, giving the growing number of patients a new treatment option.

Sample: The hygiene hypothesis states that early exposure to germs reduces the immune system’s ability to resist infections in later years. True or false?

How do we combat waning human immunity? Our slide show summarizes 5 new reports that suggest it’s essential to figure that out.

Where do US guidelines on immunotherapy for rhinitis overlap with new 2018 recommendations from Europe? Answer these 5 questions to find out.

Try this compact test on safety issues of and patieint selection for seasonal allergy medications.

What's your SLIT IQ? Find out what you know about the AAAAI guidelines on appropriate use of sublingual immunotherapy with our 5-question quiz.

Try our seasonal potpourri of questions on when to avoid skin testing, the least preferred antihistamines, the worst conjunctivitis, and best add-on meds.
What is the cause of this erythematous rash that appeared suddenly and spread rapidly on the child's trunk and extremities?
What is Dymista? Can autumn asthma be tamed? Do “wraparound” glasses work? More questions and all the answers, here.

Next time you're stumped by a difficult presentation, ask, “Have you recently started any new medications or changed the dose of any of your old medications?”

Test your intel on asthma and rain, novel immunotherapy, atopy and birth season, and other allergy aggravations.

Will steroids restore the sense of smell? Where is mold a year-round allergen? What US state makes the best home for persons with atopy? Answers and more questions in our Autumn Allergy Challenge.