Heart Failure
ABSTRACT: Diuretics remain a mainstay of heart failure therapy. Angiotensin-converting enzyme (ACE) inhibitors and ß-blockers inhibit activation of neurohormonal systems; these agents are recommended for most patients with symptomatic systolic heart failure. Angiotensin II receptor blockers (ARBs) are alternatives for patients who are unable to tolerate ACE inhibitors. Recent trials suggest that ARBs are also useful when added to the regimen of patients with a low ejection fraction. Although digoxin can provide long-term inotropic support in men, it significantly increases the risk of mortality in women; because of the risk of toxicity, use digoxin with caution in older persons and patients with renal dysfunction. Consider an aldosterone antagonist in patients who remain symptomatic at rest despite the use of a diuretic, digoxin, an ACE inhibitor or an ARB, and a ß-blocker. Lifestyle modifications such as dietary restriction and exercise are helpful in all patients.
Heart failure statistics are daunting: 550,000 new cases each year, a 1-year mortality rate of nearly 20%, and annual direct and indirect costs that total $24.3 billion.1 The diverse etiology of heart failure and the complex, progressive course of the disease can make treatment decisions daunting as well.
Here I discuss current recommendations for heart failure management, as well as the clinical implications of the results of recent studies.
RISK FACTORS
Among the 5143 participants in the original Framingham Heart and Offspring studies, the most common risk factor for heart failure was hypertension. Of those study participants in whom heart failure developed, 91% were hypertensive.2 Among hypertensive men and women in these studies, myocardial infarction (MI), diabetes, left ventricular hypertrophy, and valvular heart disease were cofactors that were associated with an increased risk of heart failure. MI was a particularly common cofactor in this group; 52% of the men with heart failure and 34% of the women had a history of MI.2
PATHOPHYSIOLOGY
The pathophysiology of heart failure involves both hemodynamic and neurohormonal abnormalities, although the interactions between them are incompletely understood. Survival benefits that result from drug-induced decreases in neurohormonal activity underscore the role of increased neurohormonal activity in chronic heart failure.
Impaired left ventricular function activates neuroendocrine compensatory mechanisms, both vasoconstrictive and vasodilative. The primary vasoconstrictive systems are the sympathetic nervous system and the renin-angiotensin system. Increased plasma levels of norepinephrine, which reflect sympathetic nervous system activity, have been correlated directly with disease severity and mortality in heart failure.3 Activation of the renin-angiotensin system increases systemic vascular resistance and has also been correlated with disease progression.
DIAGNOSIS
The history and physical findings often point to heart failure. Common presenting symptoms in patients with left ventricular dysfunction are:
- Decreased exercise tolerance (manifested by dyspnea).
- Fluid retention (manifested by swollen feet).
According to the latest American College of Cardiology and American Heart Association (ACC/AHA) guidelines, the most useful diagnostic test is the 2-dimensional echocardiogram, performed in conjunction with Doppler flow studies (Table 1).3
Much interest has recently focused on brain natriuretic peptide (BNP) as a prognostic indicator or surrogate marker.4-6 This neurohormone is secreted mainly in the cardiac ventricles. Volume expansion of the ventricles and increased intraventricular pressure raise circulating BNP levels, which rise in proportion to disease severity. The significance of this elevation depends on the patient profile (which includes systolic function and sex); thus, assessment of left ventricular function is also required.7
In a study of 78 ambulatory patients with heart failure, plasma BNP levels provided prognostic information as accurate as that derived from the commonly used multifactorial heart failure survival score.4 BNP levels were the only independent predictor of sudden death in another study population.5 A more recent study found not only that elevated BNP values correlated with first morbid event and mortality but also that percentage reduction in BNP during treatment predicted decreased risk of first morbid event and mortality.6
When performed in conjunction with echocardiography, measurement of plasma BNP concentrations may serve as a useful screening tool and thus may reduce the need for more expensive and invasive tests.4,5,7
THERAPEUTIC APPROACHES
Nonpharmacologic measures.Whether heart failure is diastolic or systolic, management always includes nonpharmacologic measures in addition to drug therapy. Discuss dietary restrictions and exercise recommendations with patients, and advise them about when to seek medical attention.
Possibly the most effective and least employed therapy is consistent attention. Follow-up and between-visit supervision by a nurse or physician assistant has yielded significant clinical benefits in symptomatic patients.3
Unfounded criteria for drug selection. The use of hemodynamic function parameters as criteria for drug selection in heart failure therapy has been based on the assumption that hemodynamic improvement will translate into symptom relief. This has proved to be an uncertain premise.8 Furthermore, the correlation between relief of symptoms and survival is also uncertain. For example, although it is well established that diuretics reduce the need for hospitalization, these agents have no apparent effect on survival. Thus, correction of a patient's hemodynamic abnormalities may not prevent heart failure progression or death.8
Current guidelines. Despite the heterogeneous etiology and complex pathogenesis of heart failure, a consensus is evolving as to what constitutes effective-and ineffective-treatment.3,9 The ACC/AHA guidelines classify heart failure into 4 stages of severity and recommend therapy targeted to each stage (Table 2). (Note that the ACC/AHA stages, which range from "at risk for heart failure" to "advanced heart failure," differ from the classes in the New York Heart Association [NYHA] grading system; the latter are based on the functional capacity of patients with heart failure.) According to the ACC/ AHA and Heart Failure Society of America (HFSA) guidelines, 4 classes of drugs should be given routinely to appropriate patients with symptomatic left ventricular dysfunction: a diuretic, an angiotensin-converting enzyme (ACE) inhibitor, a β-blocker, and (usually) digoxin (Table 3).3,9
DRUG THERAPY FOR HEART FAILURE
Diuretics. These are the most commonly prescribed agents for heart failure. Diuretics are effective in patients with diastolic or systolic dysfunction.3,9 They are indicated for reduction of the volume overload that is common in congestive heart failure, particularly in the early phase of treatment.
Digoxin. This is the only agent that can provide long-term inotropic support in men. However, digoxin significantly increases the risk of mortality in women.10
Bear in mind that the serum digoxin concentration may not correlate with the drug dosage in older persons or in patients with renal dysfunction.9 Moreover, upward titration of digoxin increases the risk of toxicity, and the beneficial effects of digoxin are not greatly enhanced at higher dosages. Additional rate control, when needed, can be provided by β-blockers or amiodarone.9
ß-Blockers.The ability of β-blockers to improve left ventricular systolic function is perhaps the most compelling evidence that chronic adrenergic stimulation damages the failing heart.9 The recent Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study reaffirms the value of this class of drugs in severe heart failure.11 Prescribe low dosages of β-blockers initially and monitor patients for worsening heart failure. After a patient has been stable for 2 weeks on a starting regimen, the dosage may be titrated upward. These agents should not be abruptly withdrawn.9 To ensure safe and effective treatment, prescribe β-blockers only for patients who:
Are clinically stable.
Have left ventricular systolic dysfunction.
Have mild to moderate symptoms.
Are receiving standard therapy.3,9
ACE inhibitors. Treatment with ACE inhibitors was the first intervention that was shown unequivocally to improve symptoms and prolong life in patients with left ventricular dysfunction. These agents counteract neurohormonal activation by lessening the deleterious effects of the renin-angiotensin system through inhibition of ACE.9 Evidence suggests that the long-term benefits of ACE inhibitors stem mainly from their ability to reverse some of the structural abnormalities associated with heart failure rather than from their hemodynamic effects.3,9 Prescribe an ACE inhibitor, together with a β-blocker and a diuretic, for patients with symptomatic systolic heart failure-unless these agents are contraindicated.3,9
Angiotensin II receptor blockers. Data now indicate that angiotensin II type 1 receptor blockers (ARBs) may confer benefits similar to those of ACE inhibitors in patients with heart failure.12 The hemodynamic actions of ARBs-eg, their reduction of systemic vascular resistance in heart failure-are comparable to those of ACE inhibitors.3,9 ARBs also improve exercise capacity and reduce norepinephrine levels in a manner similar to that of ACE inhibitors.3,9 Blockade of angiotensin II type 1 (AT1) receptors prevents the effects of angiotensin II-including that produced by enzymes other than ACE-and allows circulating angiotensin II to stimulate angiotensin II type 2 (AT2) receptors. Data suggest that some benefits of ARBs may be mediated through the AT2 receptor.13
The Valsartan Heart Failure Trial (Val-HeFT) compared the ARB valsartan with placebo in 5010 patients with NYHA class II to IV heart failure who also received standard therapy with ACE inhibitors and/or β-blockers for a mean follow-up of 23 months.12 Although overall mortality was similar, the combined end point of morbidity and mortality was 13.2% lower in the valsartan-treated group. The reduction in this combined end point resulted primarily from the 27.5% reduction in hospitalization for heart failure seen with valsartan.
In a subgroup (7% of study participants) who did not receive an ACE inhibitor, valsartan reduced all-cause mortality by 41% and hospitalization for heart failure by 57%.14 Echocardiography showed that valsartan also reversed cardiac remodeling.15 Moreover, valsartan reduced BNP levels-and demonstrated for the first time that a reduction in BNP levels during treatment is associated with a decreased risk of mortality and first morbid event.6 The data from Val-HeFT suggest that valsartan is a practical alternative to ACE inhibitor therapy.16
The Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study showed that ARBs help reduce cardiovascular morbidity and mortality. The study compared the ARB losartan (plus background therapy) with atenolol (plus background therapy) in 9193 patients who had hypertension and left ventricular hypertrophy but who did not have heart failure.17 Losartan was associated with a 13% greater reduction than atenolol in the combined end point of cardiovascular disease mortality, myocardial infarction (MI), and stroke.
The Candesartan in Heart Failure Assessment of Reduction in Mortali- ty and Morbidity (CHARM) study confirmed the benefit of ARB therapy in 7599 patients, who were stratified by ejection fraction (either greater than 40%, or 40% or less) and by whether they had previously used an ACE inhibitor.18 At a median follow-up of 38 months, the adjusted risk rate (compared with standard care) for the primary end point of all-cause mortality was 0.90 for patients who received candesartan.
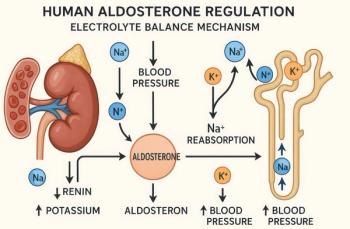
Aldosterone antagonists.Increased renin and angiotensin II levels stimulate the production of aldoste- rone, which contributes to the pathophysiology of heart failure. Elevated aldosterone levels enhance sodium retention, increase sympathetic activation and parasympathetic inhibition, and promote cardiac remodeling.3 ACE inhibitors have been assumed to suppress aldosterone production, but increasing evidence suggests that this suppression is only transient in some patients. The addition of an aldosterone antagonist such as spironolactone or eplerenone to the regimen may be helpful in such patients.
Spironolactone. In the Randomized Aldactone Evaluation Study (RALES), patients with advanced heart failure, normal serum potassium levels, and normal or mildly elevated serum creatinine levels (less than 2.5 mg/dL) received low-dose spironolactone (mean daily dose, 26 mg).19 All patients received diuretics, 95% received low-dose ACE inhibitors (mean daily dose: captopril, 63.4 mg, or lisinopril, 15.5 mg), 75% received digoxin, and 11% received β-blockers. After 24 months, the trial was terminated early because patients assigned to spironolactone had a 30% reduction in all-cause mortality.19
The reduction in morbidity and mortality among patients who were already receiving an ACE inhibitor suggests that low-dose ACE inhibition does not effectively suppress aldosterone production. Consider low-dose spironolactone for patients with severe heart failure caused by left ventricular systolic dysfunction who remain symptomatic despite optimal therapy with ACE inhibitors, β-blockers, digoxin, and diuretics.20 However, a recent analysis warns against inappropriate, indiscriminate use of spironolactone.20 The patient's heart failure classification, ejection fraction, and background treatment must be considered, and adequate follow-up must be ensured.
Eplerenone. In the recently reported Eplerenone Post-Acute MI Heart Failure Efficacy and Survival Study (EPHESUS), patients with acute MI complicated by left ventricular dysfunction and heart failure were randomized within 3 to 14 days of hospitalization to additional therapy with eplerenone, 50 mg/d, or placebo.21 Patients with elevated serum creatinine clearance (greater than 2.5 mg/dL) or elevated serum potassium levels (greater than 5 mmol/L) were excluded from the study. The majority of patients were receiving standard thera- py: ACE inhibitors or ARBs (87%), β-blockers (75%), aspirin (88%), and diuretics (60%). After a mean 16-month follow-up, all-cause mortality decreased by 15% in the eplerenone group. There was also a 13% reduction in death or hospitalization caused by cardiovascular disease. The reduction in cardiovascular disease mortality was in large part the result of a 21% reduction in the rate of sudden cardiac death.
Because of an increased incidence of serious hyperkalemia in study participants (who had been prescreened for elevated potassium levels), the investigators recommended monitoring serum potassium levels in patients being treated with eplerenone.21
HEART FAILURE THERAPY IN SPECIAL POPULATIONS
Very old patients. More than 80% of the 1 million patients hospitalized each year for heart failure are 65 years of age or older, and more than 50% are 75 years or older.22 Several studies have found nearly half of their heart failure cohort are 80 years or older.22 Advanced age complicates therapeutic decisions in several ways.
First, clinical trial data in very old patients are lacking. Despite their heavy representation in the population with heart failure, older persons are underrepresented in clinical trials of new treatments; when included, these patients are likely to be lumped together in a homogeneous "over 65" group.23
This overly broad category is problematic because the common manifestations of heart failure vary with age and sex.3 For example, diastolic heart failure becomes increasingly common with advanced age, particularly in women.3,22
Exclusion criteria used in clinical trials may further distort the study of therapy in older patients by eliminating characteristic comorbidities. In a national sample of Medicare patients, more than 55% of those with heart failure had a history of coronary disease, almost 40% had diabetes, and about 33% had chronic obstructive pulmonary disease; 10% had been admitted to the hospital from long-term care facilities.22
Furthermore, several normal aspects of the aging process complicate management. The glomerular filtra-tion rate declines with age and does so more rapidly among patients with heart failure. Consequently, a creatinine cutoff value that defines renal insufficiency for a specific medication may be inappropriately high for older persons. The effects of standard treatments at recommended dosages in older patients may differ from their effects in younger patients.
Comorbid conditions can also complicate heart failure therapy in older patients. Conditions that require medication increase the potential for adverse drug interactions.22 Moreover, the additional medications may have a negative impact on a patient's heart failure. Conversely, a comorbid condition that is not managed aggressively enough can also complicate heart failure therapy.
Patients with diastolic dysfunction. Among heart failure patients, 20% to 40% have preserved left ventricular function. For patients with systolic dysfunction, the value of various agents has been established in large-scale clinical trials. However, for those with diastolic dysfunction, large-scale trial data are lacking.3,24 Current pharmacologic treatment recommendations for patients with diastolic dysfunction are based on control of blood pressure and tachycardia, relief of myocardial ischemia, and blood-volume reduction. Treatment may include agents used for systolic dysfunction as well as calcium channel blockers and nitrates.24
References:
REFERENCES:1. American Heart Association. Heart Disease and Stroke Statistics: 2003 Update. Dallas: American Heart Association; 2002.
2. Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557-1562.
3. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. Circulation. 2001;104:2996-3007.
4. Koglin J, Pehlivanli S, Schwaiblmair M, et al. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol. 2001;38:1934-1941.
5. Berger R, Huelsman M, Strecker K, et al. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105: 2392-2397.
6. Anand IS, Fisher LD, Chiang YT, et al, for the Val-HeFT Investigators. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation. 2003;107:1278-1283.
7. Maisel AS, McCord J, Nowak RM, et al, for the Breathing Not Properly Multinational Study Investigators. Bedside B-type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. J Am Coll Cardiol. 2003;41:2010-2017.
8. Anand IS, Florea VG, Fisher L. Surrogate end points in heart failure. J Am Coll Cardiol. 2002;39: 1414-1421.
9. Heart Failure Society of America (HFSA) Practice Guidelines. HFSA guidelines for management of patients with heart failure caused by left ventricular systolic dysfunction-pharmacological approaches. Pharmacotherapy. 2000;20:495-522.
10. Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347:1403-1411.
11. Krum H, Roecker EB, Mohacsa P, et al, Car- vedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS Study. JAMA. 2003;289:754-756.
12. Cohn JN, Tognoni G, for the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667-1675.
13. de Gasparo M, Levens N. Does blockade of angiotensin II receptors offer clinical benefits over inhibition of angiotensin-converting enzyme? Pharmacol Toxicol. 1998;82:257-271.
14. Diovan [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2002.
15. Wong M, Staszewsky L, Latini R, et al. Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. J Am Coll Cardiol. 2002;40:970-975.
16. Maggioni AP, Anand I, Gottlieb SO, et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002;40: 1414-1421.
17. Dahlof B, Devereau RB, Kjeldsen SE, et al, for the LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359: 995-1003.
18. Pfeffer MA, Swedberg K, Granger CB, et al, for the CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
19. Pitt B, Zannad F, Remme WJ, et al, for the Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-717.
20. Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. J Am Coll Cardiol. 2003;41:211-214.
21. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321.
22. Havranek EP, Masoudi FA, Westfall KA, et al. Spectrum of heart failure in older patients: results from the National Heart Failure project. Am Heart J. 2002;143:412-417.
23. Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162: 1682-1688.
24. Zile MR, Brutsaert DL. New concepts in dia- stolic dysfunction and diastolic heart failure, II: causal mechanisms and treatment. Circulation. 2002;105:1503-1508.
Newsletter
Enhance your clinical practice with the Patient Care newsletter, offering the latest evidence-based guidelines, diagnostic insights, and treatment strategies for primary care physicians.