Essential Resources
Provider Information
Patient Information

Evidence for Adverse Effects of High-Dose GC Treatment of Congenital Adrenal Hyperplasia Broadened, Deepened in New Literature Review
The most commonly reported adverse effects of high-dose glucocorticoid therapy were on bone health, cardiometabolic function, and height and growth.
Crinecerfont Reduces GC Dosing, Improves Androstenedione Levels Across Multiple Pediatric Subgroups
Data for GC reduction and improved A4 levels were consistent across subgroups stratified by sex, race, age, BMI, and baseline A4 levels, according to Neurocrine Biosciences.
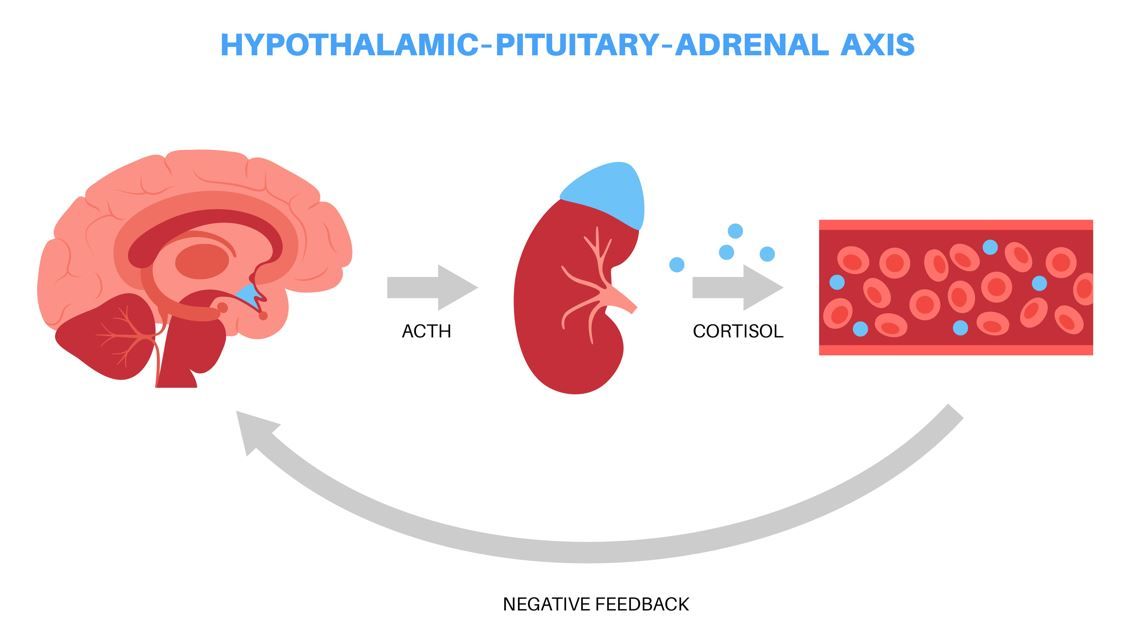
Congenital Adrenal Hyperplasia Management Across the Lifespan
The cortisol deficiency central to congenital adrenal hyperplasia requires lifelong treatment with distinct shifts in management required at each developmental stage.
The CRF Pathway Interrupted: Potential to Transform CAH Care
A therapeutic strategy that reduces ACTH-driven androgen excess through a glucocorticoid-independent pathway could significantly improve treatment and patient QoL.
The CAHtalyst Adult Phase 3 Clinical Trial of Crinecerfont: Principal Investigator Interview
More than 6 in 10 adults treated with the investigational CRF1 antagonist crinecerfont reached a physiologic glucocorticoid dose with androstenedione control maintained.
The Ideal Treatment for CAH? We Asked the Expert
Dr Richard Auchus says the ideal treatment for congenital adrenal hyperplasia is a long way off but describes a "block and replace" strategy that holds great promise.
Crinecerfont Could Help Improve Physical, Emotional Health for Individuals with CAH
Crinecerfont, a CRF1 antagonist, could help reduce glucocorticoid doses for individuals with CAH and improve physical and emotional quality of life, says leading researcher.
A Primer on Congenital Adrenal Hyperplasia with Expert Steroid Biologist Richard Auchus, MD, PhD
Richard J Auchus, MD, PhD, a preeminent steroid biologist, provides a concise and thorough primer on CAH and the challenges in managing the disorder safely and effectively.
Monitoring Glucocorticoid Therapy in CAH: Timing, Timing, Timing
Clinical guideline recommendations for optimal timing to gauge glucocorticoid treatment effects in congenital adrenal hyperplasia require individualization. Here's why.
Treating Congenital Adrenal Hyperplasia: Challenges in the Transition to Adult Care
In young adults with congenital adrenal hyperplasia of age to transition to adult care, 25% to 50% are not successful. Here are thoughts on problems and solutions.
FDA Accepts 2 NDAs, Grants Priority Review for Crinecerfont for Pediatric, Adult CAH
Neurocrine Biosciences made the announcement and said, if approved in December, crinecerfont will be the first new treatment and treatment approach to CAH in 70 years.
Management of Congenital Adrenal Hyperplasia in Adults: Challenges with Standard of Care
The standard of care for congenital adrenal hyperplasia has not changed in more than 60 years nor have the challenges it presents been overcome.
Emerging Therapies in Management of Congenital Adrenal Hyperplasia
Investigational treatments for congenital adrenal hyperplasia are focused on new ways to manage symptoms without the need for supraphysiologic doses of glucocorticoids.
Androgen Management in Congenital Adrenal Hyperplasia: A Primer
Current therapies fall short of quelling the long-term exposure to and adverse effects of excessive androgen levels experienced by adults with CAH.
Neurocrine Biosciences CAHtalyst Adult Phase 3 Clinical Trial Meets All Endpoints in Congenital Adrenal Hyperplasia, Published in NEJM
The registrational clinical trial met all endpoints related to androgen reduction and glucocorticoid dose reduction while maintaining androgen control.
FDA Grants Breakthrough Therapy Designation for Oral Congenital Adrenal Hyperplasia Medication
Crinecerfont was granted FDA breakthrough therapy designation for the treatment of congenital adrenal hyperplasia.