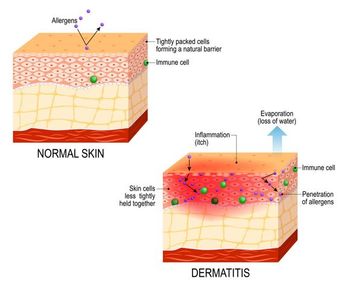
Atopic Dermatitis Treatments Up Close: Biologics, JAK Inhibitors, and Cytokines, Oh My
Atopic dermatitis management will be the topic of a debate over treatment with biologics and JAK inhibitors and in a focused session on the role of IL-13 in the chronic skin disease.
A Saturday afternoon session at the Winter Clinical Hawaii Dermatology Conference will focus on the features and benefits of 2 contemporary classes of drugs to treat
The debaters, Neal D. Bhatia, MD, director of Clinical Dermatology Therapeutics Clinical Research in San Diego, CA, and Raj Chovatiya, MD, PhD, assistant professor, director of the Center for Eczema and Itch and medical director of clinical trials at Northwestern University Feinberg School of Medicine in Chicago, IL, will begin their conversation with a patient case and then provide evidence for their recommendations.
While the details of their specific points of view aren't known yet, a few of the possible topics for discussion follow here. The classes differ in a number of important ways that will affect not only clinical patient selection but patient and caregiver preference as well.
Biologic agents modulate the type 2 inflammatory cascade through targeted inhibition of specific cytokines including interleukin (IL)-4, -13, and most recently IL-31.
Janus kinase (JAK) inhibitors impede JAK-STAT signaling pathways and provide broad immunomodulation across the same cytokines targeted by the biologic drugs. The JAK inhibitors are associated with more rapid onset of action than the biologics, with symptom relief often occurring within days of treatment initiation and they also have shown higher rates of early skin clearance in clinical trials.1 There are 3 JAK inhibitors approved for treatment of AD:
The key difference between the classes is their relative safety. JAK inhibitors carry an FDA black box warning for serious infections, thromboembolic events, and malignancy.2 Patients using a JAK inhibitor need to be monitored for anemia and changes in lipid levels.3 The safety of the drugs for long-term use is a concern and particularly for older or high risk individuals. Only safety data for administration of JAK inhibitors for approximately 1 year has been published.1
On the other hand, there are concerns about the potential for immunogenicity with the biologics,4 although incidence in clinical trials was quite low (less than 0.6% of participants).1
The debaters on Saturday will go into more detail on the features and benefits and pros and cons associated with each of the classes including patient selection and preference. The literature suggests that ideal candidates for biologics have type-2 inflammation-driven AD, are interested in long-term maintenance therapy, and have lower risk tolerance for systemic immunosuppression. Patients more suited to JAK inhibitors may be in need of rapid control of severe disease flares, be non- or partial responders to biologic agents, and/or prefer oral or topical therapy to injections.
Ultimately any debate over which class or which drug within a class will take the form of noncontentious shared decision making session between a clinician and a patient that will consider an individual's age, AD severity, comorbidities, and personal preferences.
Find out where Drs Bhatia and Chovatiya net out on the topic by logging in by registering for virtual access to the Midwinter Clinical or by checking back to our conference coverage page for our recap of the conversation.
References
1. Kamata M, Tada Y. J Optimal use of Jak inhibitors and biologics for atopic dermatitis on basis of current evidence. ID Innov. 2023;(3):100195. doi: 10.1016/j.xjidi.2023.100195
2. Janus Kinase (JAK) inhibitors: Drug Safety Communication - FDA Requires Warnings about Increased Risk of Serious Heart-related Events, Cancer, Blood Clots, and Death. Medical products safety information. US Food and Drug Administration. September 1, 2021. https://www.fda.gov/safety/medical-product-safety-information/janus-kinase-jak-inhibitors-drug-safety-communication-fda-requires-warnings-about-increased-risk
3. Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047–1055. doi: 10.1001/jamadermatol.2021.3023.
4. Immunogenicity of dupilumab in adult and pediatric patients with atopic dermatitis. Front Immunol. 2024;15. doi:10.3389/fimmu.2024.1466372
Newsletter
Enhance your clinical practice with the Patient Care newsletter, offering the latest evidence-based guidelines, diagnostic insights, and treatment strategies for primary care physicians.